Designing extra hospitality in medical center. City design alternatives are not a heal-all On the subject of rural Health care desires.
No matter these tactics, the aptitude of the process to produce sterile goods have to be validated to operate in accordance with pre-founded requirements.
We also offer demonstration models to test, outline operational processes about, and approach workflows. Contact us to find out The ultimate way to aid your challenge requirements.
The exposure of open agar-crammed Petri dishes, or settling plates, will not be to be used for quantitative estimations on the microbial contamination levels of critical enviroments.
Preferably a combination of airlocks and room pressurization produce a much better defense to possible contaminants. Within the design of latest facilities, the process devices is usually designed as the primary degree security for your product by using closed techniques for transferring components from just one container to another or from containers in to the products.
The mixing of automation with cleanroom sterilization products is likewise enhancing the opportunity to acquire and examine knowledge. This qualified prospects to higher system understanding and facilitates constant improvement.
Pharmaceutical items, notably sterile drugs and biologics, demand an setting freed get more info from contaminants to forestall probable hurt to patients and ensure the efficacy of your merchandise.
Waiting for 2025, we are able to count on to find out more harmonization of regulatory requirements throughout distinct areas, in addition to a heightened target the validation of novel sterilization systems.
Microbiological checking of personnel is additionally being incorporated into teaching plans. Frequent tests of gloves and gowns making use of Call plates or swabs supplies quick feedback on the efficiency of aseptic tactics and will help reinforce good tactics.
Staff schooling is a vital part of maintaining sterility in pharmaceutical cleanrooms. As cleanroom technological innovation and sterilization procedures evolve, so much too ought to the methods to instruction cleanroom staff.
The evaluative means of clean room fumigation techniques is rather rigorous and scientific as it should be Licensed that item security is ensured. This can easily be accomplished as a result website of adherence to established common procedures, introduction of latest fumigants, and common compliance, which are meant to be adopted because of the pharmaceutical firms so as to scale back contamination.
Media fill packages should also simulate creation methods more than prolonged operates. This can be completed by doing media-fill runs at the conclusion of production runs.
by keeping contaminants from injections, drops, along with other sterile compounding preparations; While, USP 800 regulations emphasize employee safety
Custom-designed cleanroom elements with optimized surfaces for cleanliness and sterilization can now be created on-demand, bettering the overall design and features of cleanroom environments.
 Jonathan Taylor Thomas Then & Now!
Jonathan Taylor Thomas Then & Now! Jenna Jameson Then & Now!
Jenna Jameson Then & Now!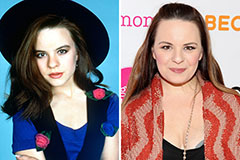 Jenna Von Oy Then & Now!
Jenna Von Oy Then & Now! Kelly Le Brock Then & Now!
Kelly Le Brock Then & Now! Matilda Ledger Then & Now!
Matilda Ledger Then & Now!